FDA gives nod to first fully-removable percutaneous peripheral
$ 79.50
-
By A Mystery Man Writer
-
-
4.7(228)

Product Description
SPR Therapeutics has developed a peripheral nerve stimulation system that is placed percutaneously through the skin instead of being implanted and which can be completely removed from the body after therapy period.

Latest News Archives - Interventional News
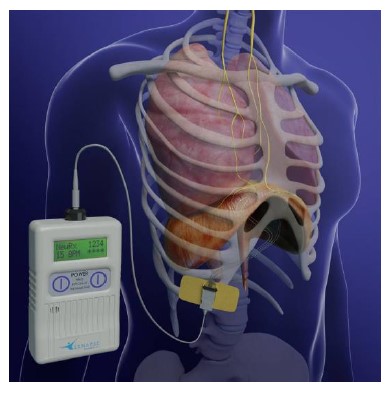
NeuRx Diaphragm Pacing System – P200018

urmila, Author at Interventional News

A) The percutaneous PNS therapy uses fine-wire, coiled leads typically

Locoregional delivery of CAR-T cells in the clinic - ScienceDirect

NEUSPERA MEDICAL® ANNOUNCES FDA CLEARANCE OF ITS SYSTEM FOR PERIPHERAL NERVE STIMULATION

SCV News FDA: Drug Safety Updates & Approvals
Arterial puncture site management